Augite - Encyclopedia
Augite - Encyclopedia
Class : Silicates
Subclass : Inosilicates
Crystal system : Monoclinic
Chemistry : (Ca, Mg, Fe)2 (Si, Al)2O6
Rarity : Very common
Augite is the most common pyroxene in the earth's crust. It is an essential constituent of basic magmatic rocks (andesites, basalts, gabbros, etc...) and ultrabasic (pyroxenites, etc...), which one also meets in metamorphic rocks of high temperature (granulite) and of contact. Its name comes from the Greek auge (shiny), alluding to the appearance of its cleavage surfaces. The chemistry of augite is complex : it forms a continuous series with diopside and hedenbergite, by replacing atoms of silicon and magnesium by atoms of aluminum ; in addition, the presence of titanium and chromium is frequent, especially in volcanic environments. The assimilation of sodium also allows the formation of terms of passage towards aegyrine : these are the aegyrinic augites. A frequent phenomenon is the alteration of augite to green hornblende ("ouralitization"). Well crystallized, augite shows crystals with various facies, elongated or squat with square or octagonal section, frequently twinned (main photo). Its color is black with brownish to greenish reflections. It is a mineral that has no particular use.
Augite in the World
Augite in France
In France, the volcanoes of the Massif Central have yielded superb individuals : 6 cm in Mézenc (Haute-Loire), more than 3 cm in Cantal and Ardèche. In Puy-de-Dôme, augite is very abundant in the pozzolana of Puy de Corent (photo on the right), although automorphic crystals are rare. It is found in alluvium of volcanic products from the Chaîne des Puys, at Aydat for example in 5 mm perfect and twinned crystals.
Twinning
Single or multiple twins on {100} (in bishop's bonnet) and more rarely on {001} are known. In addition, orthopyroxenes such as enstatite may exhibit clinopyroxene exsolutions (augite) just as augite may exhibit orthopyroxene exsolutions. These oriented exsolutions are often confused with twins.
On the left, a photo of an orthopyroxene exsolution in augite (yellow bands) in analyzed-polarized light.
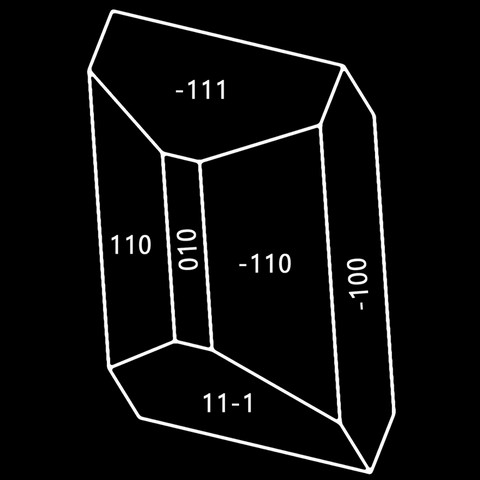
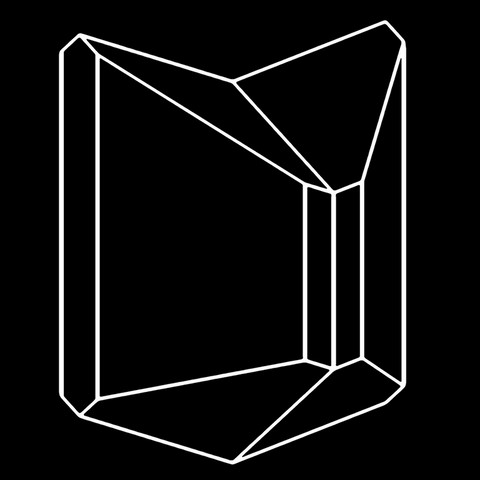
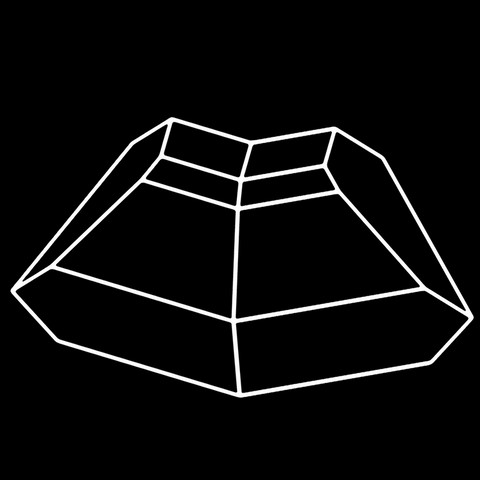
The different crystal forms of augite
Fakes and treatments
No fake recorded for this mineral species.
Hardness : 5.5 to 6
Density : 3.19 to 3.56
Fracture : Irregular
Trace : Green to brown
TP : Translucent to opaque
IR : 1.680 to 1.774
Biréfringence : 0.032
Optical character : Biaxial +
Pleochroism : Visible
Fluorescence : None
Solubility : Hydrofluoric acid
Magnetism : Paramagnetic
Radioactivity : None