CALCITE
Class : Carbonates, nitrates, borates, iodates
Subclass : Anhydrous carbonates
Crystal system : Trigonal
Chemistry : CaCO3
Rarity : Very common
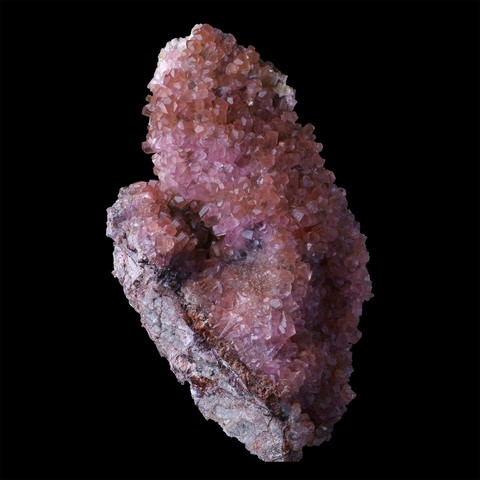
Calcite is the most common of carbonates. It is found almost everywhere, in very various geological contexts : sedimentary carbonate rocks, karstic environments (caves) and metamorphic rocks derived from sediments (marbles), hydrothermal in metalliferous veins (associated with fluorite, quartz, baryte and many others sulphides) but it can also be of magmatic origin (in cavity of basaltic lavas, but also the mineral of carbonatitic lavas). Its name comes from the Latin calx (lime) of which it is the base of production. Its colors can be varied according to the impurities it contains, usually colorless to white when it is pure, it can also be honey yellow to brown, sometimes pink when it contains cobalt (cobaltoan calcite), mauve with manganese (manganoan calcite), blue or green in presence of copper. The crystalline forms of calcite are extremely numerous, there are more than 700 different facies : rhombohedral with all possible combinations (acute or obtuse rhombohedra, elongated prisms or very flattened tablets, scalenohedra often modified, etc...). Calcite is an important industrial mineral, it is the basis of many cements and lime, it serves as mineral filler in the paper industry, paints and coatings, plastic, etc... It is also used extensively in agriculture for the amendment of acid soils. It can be cut for jewelery, but its low hardness makes it useless. In transparent quality it has however much used in optical instruments as a source of polarized light.
Calcite in the World
Calcite in France
In France, calcite is very present. Among the most beautiful crystals we will mention the carboniferous limestones exploited at Louverné (Mayenne), while in the formation of the Fontainebleau sandstones, the calcite of Bellecroix is an original facies consisting of calcite rhombohedra impregnated with grains of siliceous sand. Also worth noting are the spectacular diamond calcites of the Jurassic conglomerates of the Pau region (photo on the right).
Twinning and special crystallisations
The calcite presents numerous union twinning of which a very common polysynthetic twin on {01-12}. Among the most spectacular are the heart twin on {10-11} and the butterfly wing twin on {02-21} (photo on the right shows that twin on a Chinese specimen).
Calcite can also replace many other mineral species, among the most beautiful classics Russian ikaite crystals. It is also found as a replacement for some fossils including Malagasy ammonites but also in geode in the bivalves of Ruck's Pit, Florida.
Fakes and treatments
Among the classic scams, there is the copper sulphate dyeing, especially to obtain green crystals. We can potentially have flashy pink surface coating to mimic cobaltoan or manganoan calcite, and we potentially find any other eccentric colors.
In conventional treatment, it is also necessary to point out the acidification. In order to improve the surface condition of corroded or encrusted crystals, the samples are sometimes immersed in a rapid bath of hydrochloric acid, which gives the specimens a "melted" glossy appearance. It should be noted that acid or oil may be applied on the impacts or damage of the crystals to hide them.
Hardness : 3
Density : 2,6 to 2,8
Fracture : Spathic to conchoidal
Trace : White
TP : Transparent to opaque
RI : 1,64 to 1,66
Birefringence : 1,54 to 1,74
Optical character : Uniaxial -
Pleochroism : Various
Fluorescence : Red, white, blue, green
Solubility : Hydrochloric acid
Magnetism : None
Radioactivity : None