AMBER
Class : Organic compounds
Subclass : -
Crystal system : Amorphous
Chemistry : (C, H, O)
Rarity : Rare to common
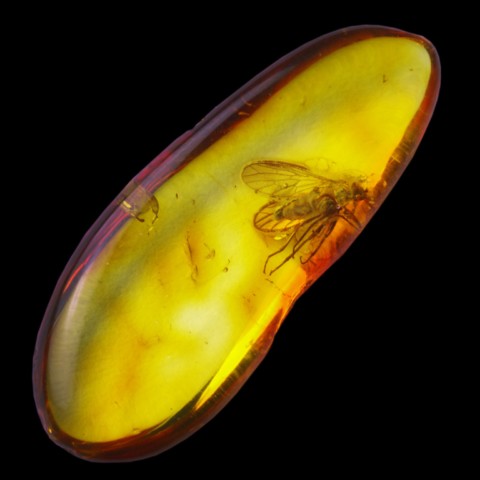
Amber is most often the fossilized resin of a pine from the Tertiary era (Pinus succinifer) which has acquired over time a crystalline structure and a chemical composition sufficiently constant to be ranked among the minerals. It can also be much older, the oldest amber discovered date back to 320 million years (Carboniferous). It has been known since very ancient times, there are artefacts carved in amber dating back more than 10,000 years before our era. Its current name comes from the Arabic anbar (grey amber), the Greeks called it electrum ; the resulting word "electricity" refers to its electrical properties : by friction amber loses electrons and becomes electrostatic. Historically, people also burned amber like incense : when it burns, it gives off a sweet smell. The German word for amber is “bernstein”, which literally means “to burn stone”. It must be at least a million years old to be called amber. Otherwise, it is called copal, or "immature amber". Copal is softer than amber and melts at a lower temperature. Amber being of plant nature, it is quite naturally most often associated with fossil fuels (coal and lignite). However, it is mainly exploited in fluvial and coastal detrital deposits. Amber never appears there in crystals but in gravels and masses, weighing up to 10 kg (Baltic) sometimes 20 kg (Myitkyina, Myanmar). The colors of amber are variable, classically yellow or yellow-orange, it can become darker with oxidation and UV to become brown or even reddish, specimens with extreme fluorescence can come out blue or green. Samples of great clarity are the most sought after for jewelry and scholars since Pliny the Elder have been interested in them. Amber also owes its reputation to the "mummified" insects it can contain. The film "Jurassic Park" also propelled this mineral to the fore, which has since considerably ignited public interest in this stone. Besides the grand spectacle aspect, the animals often beautifully preserved prisoners of this resin are the object of intense paleontological research.
Amber in the World
Amber in France
In France, a few small occurrences exist but are only simple curiosities. We can cite Duilhac-sous-Peyrepertuse near Narbonne (Aude) in the Upper Turonian, La Garnache (Vendée) or even the gypsum quarry of Arpenty (Essonne). In the terminal Albian of Archingeay in Charente-Maritime, amber contains insects and plants.
Fakes and treatments
Many treatments are done to improve the appearance of amber and its merchantability. They can clarify cloudy amber slightly when it is carefully heated in colza or canola oil. The oil penetrates and fills the bubbles which cause the opacity. The treatment is similar to emerald oil to minimize the visibility of inclusions. The resulting amber sometimes presents circular marks reminiscent of discoidal fractures and called "sun spangles".
Treatments can also change the yellow color of amber to a yellowish-brown color that is sometimes reddish and simulate what is called an "aged" color. Amber can also be dyed to produce other colors, especially green, or heat it to darken its appearance.
Manufacturers can create large chunks of amber by pressing small, gem-quality chunks or shavings under gentle heat or pressure. The resulting product is sometimes referred to as ambroid, pressed amber, consolidated amber, reconstructed or reconstituted amber. You can identify the ambroid under magnification by finding margins that vary in clarity or bubbles that have been elongated or distorted during the manufacturing process.
Amber has a host of natural and artificial imitators. Bakelite, a hard plastic, is probably the most common artificial imitator. Casein, celluloid, epoxy, glass, and polyester are some of the other imitators. Many imitations of amber also contain insects, artfully inlaid by hand.
Hardness : 2 to 2.5
Density : 1.05
Fracture : Conchoidal
Trace : White
TP : Translucent to transparent
RI : 1.54
Birefringence : 0
Optical character : None
Pleochroism : None
Fluorescence : Green to blue
Solubility : None
Magnetism : None
Radioactivity : None