FERRIERITE
Class : Silicates
Subclass : Tectosilicates
Crystal system : Orthorhombic
Chemistry : (Na,K)2MgAl3Si15O36(OH) 9H2O
Rarity : Rare
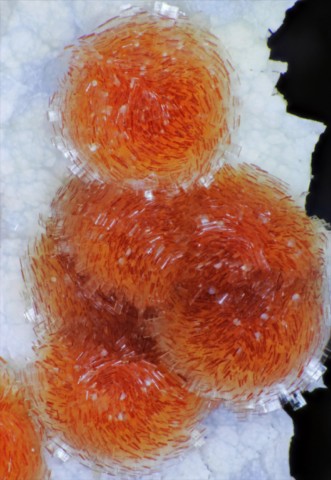
Ferrierite belongs to the zeolite group. It is a rare species which fills cracks and cavities in basalts and andesites, as well as interstices in rhyolitic tuffs, associated with calcite and other zeolites. It was named in honor of the Canadian geologist and mining engineer Walter Frederick Ferrier. Ferrierite occurs in lamellar to tabular crystals, frequently elongated along [001] and forming radiate groups that are sometimes spherolitic. They are usually small (0.5 to 1 cm) but can exceptionally reach 8 cm. Ferrierite is colorless, white to pink, sometimes salmon pink. it has one of the smallest densities of zeolites (2.1).
Main photo : Ferrierite from Kamloops Lake, British Columbia, Canada © Rudy W. Tschernich
Ferrierite in the World
Twinning
No twin known for this mineral species.
Fakes and treatments
No fakes recorded for this mineral species.
Hardness : 3 to 3.5
Density : 2.13
Fracture : Irregular
Streak : White
TP : Translucent to transparent
RI : 1.473 to 1.492
Birefringence : 0.004
Optical character : Biaxial (+/-)
Pleochroism : None
Fluorescence : White-blue
Solubility : Hydrochloric acid
Magnetism : None
Radioactivity : None