MIRABILITE
Class : Sulfates, chromates, molybdates
Subclass : Hydrated sulfates
Crystal system : Monoclinic
Chemistry : Na2SO4 10H2O
Rarity : Common
Mirabilite (Glauber's "admirable salt") is mainly formed by the evaporation of saline solutions : it is therefore mainly an evaporitic mineral from salt lagoons in arid regions, but which is also found in the fumaroles of hot springs. Its solubility decreasing sharply with temperature, mirabilite is often deposited during the cold season along with gypsum, thenardite and glauberite. Its name comes from the expression "sal mirabile" (wonderful salt) given by the German chemist J.R. Glauber who discovered it. Mirabilite is a very fragile mineral because of its high solubility in water and its easy dehydration in a dry atmosphere. Usually transparent and colorless, it becomes white and opaque on exposure to air. It most often constitutes efflorescent encrustations, stalactites or crystalline masses ; the crystals are elongated and flattened, fibrous to acicular, sometimes short prismatic and then resembling those of pyroxenes or borax. It is a secondary sodium ore.
Main photo : Minyulite from Tom's Quarry, Kapunda, Australia © Stephan Wolfsried
Mirabilite in the World
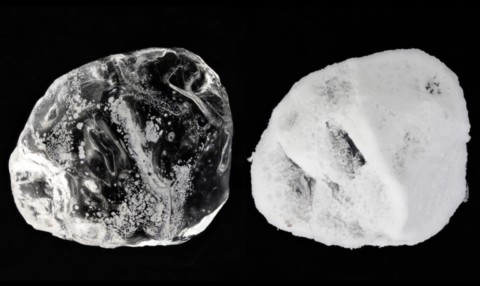
Twinning
Interpenetration twins are known on {100} and on {001}.
Fakes and treatments
No fakes recorded for this mineral species.
Hardness : 1.5 to 2.5
Density : 1,464
Fracture : Conchoidal
Streak : White
TP : Transparent to opaque
RI : 1.391 to 1.398
Birefringence : 0.005
Optical character : Biaxial -
Pleochroism : None
Fluorescence : None
Solubility : Water
Magnetism : NoneRadioactivity : None

