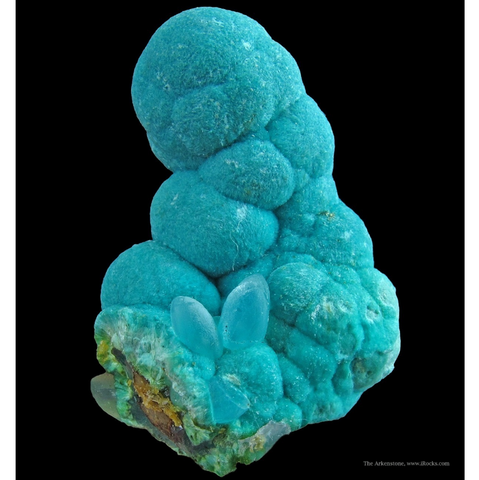
AURICHALCITE
Class : Carbonates, Nitrates, Borates
Subclass : Anhydrous carbonates
Crystal System : Monoclinic
Chemistry : (Zn,Cu)5(CO3)2(OH)6
Rarity : Uncommon
Aurichalcite is a secondary carbonate produced in hot climates, in the oxidation zones of copper and zinc deposits. Crystallographically, it is the copper equivalent of hydrozincite. Its name comes from the Latin aurichalcum (yellow copper) and the Greek orikhalcos (copper ore). It comes in fine flattened and striated crystals, most often acicular, assembled in delicate tufts, feather dusters or fragile fibrous encrustations on a matrix of limonite ; it is more rarely columnar, grainy, botryoidal or scaly. Under its most characteristic facies it is translucent, has a silky to pearly luster, and a magnificent pale green to greenish blue, sky blue or turquoise blue color. The crystals hardly reach the centimeter.
Main photo: Aurichalcite from Rohdenhaus Quarry, Germany © Harjo Neutkens
Aurichalcite in the World
Twinning and special crystallizations
No twin known for this mineral species.
Fakes and treatments
No fake or treatment identified for this mineral species.
Hardness : 1 to 2
Density : 3.96
Fracture : Irregular
Trace : Light blue
TP : Transparent
RI : 1.655 to 1.744
Birefringence : 0.089
Optical character : Biaxial -
Pleochroism : Low
Fluorescence : None
Solubility : Acids, ammonia
Magnetism : None
Radioactivity : None