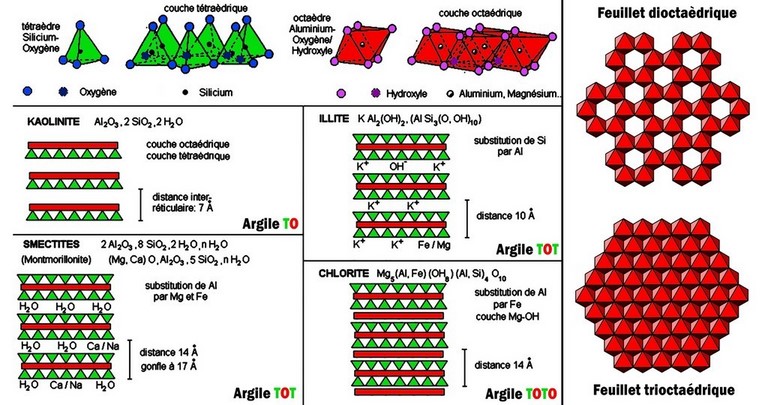
CHLORITE
Class : Silicates
Subclass : Phyllosilicates
Crystal system : Monoclinic
Chemistry : A5-6Si4O10(OH)8
Rarity : Very rare
Chlorites are a group of magnesium, iron and aluminum phyllosilicates. They owe their name to their color khlôros meaning "green" in Greek. The crystalline structure of chorites is close to that of micas, but the sheets (of the "talc" type) are separated not by alkaline ions (K, Na) as in micas, but by trioctahedral sheets Mg(OH)2 ("brucite" type). This sheet structure is responsible for the perfect cleavage (see illustration below). The chemistry of chlorites is complex, especially as there are transition terms with illite, montmorillonite or vermiculite : these are interstratified. Currently the international nomenclature recommends distinguishing 4 main mineral species among the chorites : clinochlore (magnesium), chamosite (ferriferous), these two minerals forming a continuous solid solution, pennantite (manganiferous) and cookeite (lithiniferous). The varieties are also numerous (pennine, ripidolite, sheridanite, brunsvigite, etc...). Chlorite occurs as squat, pseudohexagonal lamellar crystals, flattened on {001}, sometimes taking the appearance of barrels or prisms with horizontally striated faces. Sector twins are not uncommon. It also occurs in foliaceous, vermicular, or scaly aggregates and in compact masses, finely crystalline to cryptocrystalline. The color is usually dark green to greenish black, exceptionally lavender (chromiferous varieties). It is a very common group in low-gradient metamorphic rocks (chlorite schists, zeolite facies), as well as in altered magmatic rocks (basalts, spilites, gabbros, etc...), and alteration aureoles around hydrothermal deposits. (so-called "propylitic" alteration). In these alteration contexts, chlorite comes from the transformation of ferromagnesian minerals (biotite, amphibole, pyroxene).
Hardness : 2 to 2.5
Density : 2.6 to 3.3
Fracture : Scaly
Trace : White to grey
TP : Opaque to transparent
RI : 1.57 to 1.67
Birefringence : 0.005 to 0.011
Optical character : Variable
Pleochroism : Visible
Fluorescence : None
Solubility : Insoluble
Magnetism : None
Radioactivity : None