DIAMOND
Class : Element
Subclass : Non-metals
Crystal system : Cubic
Chemistry : C
Rarity : Rare
Diamond is the hardest of all known natural bodies (hardness of 10 on the Mohs scale). Its name comes from this hardness : from the Greek adamas (invincible). Combined with an exceptional refractive index at the origin of the luster (adamantine) and its lights, they make diamond a legendary mineral, the most prized of precious stones. Diamond is a primary mineral brought up from the deep mantle by kimberlites, these ultrabasic volcanic rocks very rich in phlogopite and pyrope, which are put in place in the form of particular volcanic chimneys : "the pipes". Diamond is also found in detrital deposits deriving from the alteration of kimberlites (conglomerates alluvium). Crystals are very common. Most frequently they are octahedra (up to 6 cm), sometimes dodecahedra, tetrahedra or cubes, with faces often striated and curved. Only transparent and flawless diamonds, colorless or harmoniously colored in yellow, red, blue or orange are cut for jewelry : they remain in the minority compared to impure diamonds, dark in color, the "borts", in great demand in cutting industries, and partially produced by synthesis to compensate for the low level of mining production. Indeed, diamond saws have a steel crown set with fragments of diamond crystals in order to cut glass, rocks and hard materials, it is again fragments of diamonds that allow drill bits to dig rocks, to achieve the wire drawing or truing of the grinding wheels. Diamond powder is used for polishing the diamonds themselves but also other gemstones. In jewelry, its price depends on its color, its brilliance, due to its high refractive index, and its fires, which reflect its strong dispersion of light. Diamonds colored in yellow, blue, green or red are the most popular and fetch sky-high prices.
The diamond is so precious that it is deified in the marvelous religious : for the Hindu Vedas "the diamond throne of Buddha is at the center of the World", while the Apocalypse of St. John proclaims that "the white throne of judgment last is cut in the diamond". India, cradle of precious stones, will remain with Borneo the only diamond producer in the world until the 18th century. It was a very precious talisman : Indian lapidaries claimed that the diamond owner was protected from snakes, fire, disease, poisons, thieves, ghosts and floods. The Romans did not hold the diamond in great esteem, relegating it to the rank of tool for cutting "really" precious stones, mainly emerald.
Diamond in the World
The de Beers brothers were the proud owners of this diamond-bearing strip of land that would become the De Beers Mine. The kimberlite "pipes" were then actively researched and intensely exploited. The town and suburbs of Kimberley have five of the most famous and productive in the world : Kimberley Mine, known as the "big hole", one of the largest man-made excavations, De Beers mine, Bultfontein, Dutoitspan and Wesselton. These five mines together produced between 1888 and 1987 nearly 22.5 tonnes of diamonds (over 112 million carats). But it is another kimberlite, mined in the Premier Mine, which produced the most beautiful stones, including the famous Cullinan, the largest diamond in the world. With a gross weight of 3106 carats (621.2 g or the size of a large matchbox), this gem was discovered on January 5, 1905 and purchased from Thomas Cullinan by the Transvaal government to be offered to the British crown. The Cullinan was cut into 9 large brilliants and 96 brilliants of smaller sizes ; the largest, the "Great Star of Africa", weighing 530.2 carats, adorns the scepter of British sovereigns. The Premier mine produced more than 300 stones of very high quality exceeding 100 carats, including in 1985 a yellow diamond with a gross weight of 755 ct : the Golden Jubilee. The latter was cut from a 545.67 ct stone, currently the largest cut diamond in the world. This very rich kimberlite is also the oldest : it has been dated to 1.2 billion years old.
Superb diamonds also come from India such as this brown stone of 1.25 ct (the Golconde alluviums were the only world producers until the 18th century), Brazil (Diamantina), the Democratic Republic of Congo and Yakutia. (Russia).
Annual global mining production stood at 130 million carats (or 26 tonnes of diamonds) in 2016, with Congo and Botswana being the world's leading producers ahead of Russia and South Africa. But this production is not enough to meet industrial demand and diamonds have been synthesized since 1954.
Diamond in France
In France, the diamond is reported in the komatiites of the Dachine complex in French Guyana. It is also found in certain meteorites including the Orgueil meteorite, which fell on May 14, 1864, the most massive of the 9 known carbonaceous chondrites.
Twinning
Diamond twinned on {111} is the spinel twin law. It usually produces flattened triangular crystals (photo on the right). This twin can exceptionally be multiple and produce stars of David (photo on the right) just like the spinel.
The diamond can more rarely present a twin identical to that of fluorite which associates two interpenetrating individuals with ideally 18 re-entrant angles. We also speak of a fluorite twin (diagram on the right).
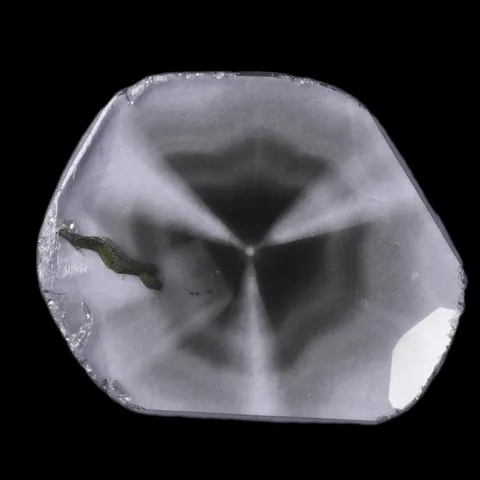
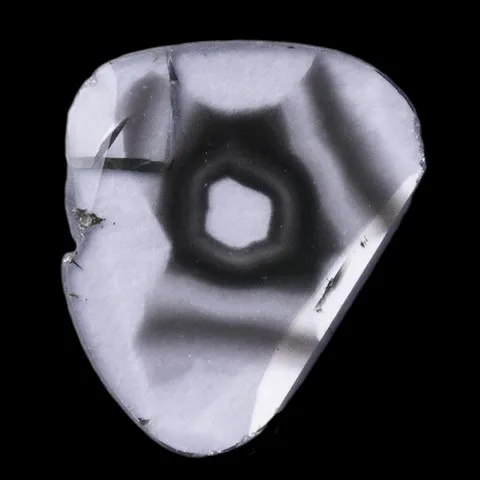
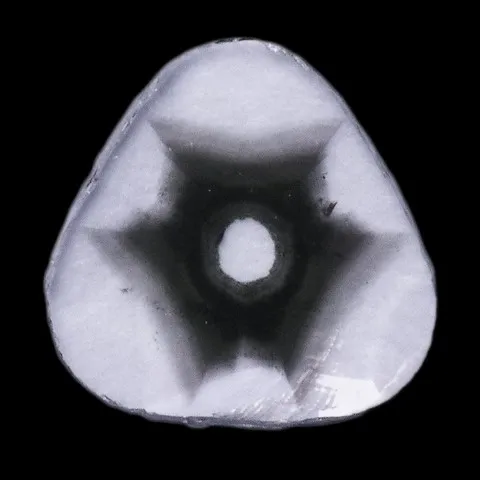
Trapiche-like diamonds
In the early 2010's, diamonds with gray-black zoning were discovered in Zimbabwe. These are graphite inclusions oriented along certain crystallographic faces of the diamond. Fine polished slices were made in order to highlight these zoning in the same way as we find polished tourmaline slices. This rare feature has been wrongly called "trapiche" although it is simply a question of zoning.
Some exceptional stones have been cut for jewelry like these superb octahedra presented on the right.
To read : Trapiche-like diamond from Zimbabwe
Synthetic diamonds

Most synthetic diamonds are made at high pressure. They are primarily intended for industrial use, but some manufacturers produce them for jewelry. Nitrogenous impurities usually color synthetics brown or yellow as they grow, so colorless synthetic diamonds are rare. Magnification and illumination against a black background can reveal opaque or reflective metallic flux inclusions in a synthetic diamond. These inclusions do not appear in all stones.
A synthetic diamond produced by conventional high pressure synthesis grows from a seed crystal and has octahedral and cubic faces. This means that a well-formed crystal looks like a tapered pyramid with a wide base with a small, flat face at the top (pictured right). Natural diamond crystals (which grow as octahedra or cubes) look very different.
Yellow synthetic diamond crystals contain nitrogenous impurities. When the impurities line up with the octahedral and cubic faces of the crystal, they can create a characteristic hourglass-shaped or cross-shaped internal grain that can be seen in gems.
Most colorless to nearly colorless synthetic diamonds are devoid of nitrogen impurities, so they generally have no internal or surface grain. It is possible to see clouds of tiny point inclusions similar to those found in natural diamonds, but they can be very difficult to diagnose and are not a conclusive way to separate natural diamonds from their synthetic counterparts.
Colorless to nearly colorless synthetic diamonds generally exhibit weak double refraction (ADR). It sometimes appears as a black cross-shaped pattern under crossed polarizing filters (polarized-analyzed light) as shown in the photo.
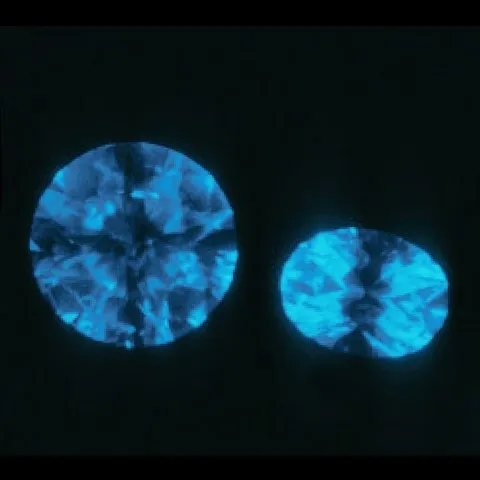
Most colorless synthetic diamonds react more strongly to shortwave ultraviolet (UV) rays than to longwave UV rays. Under shortwave UV, they may show weak to strong yellow, green-yellow, or orange-yellow fluorescence. If natural diamonds are fluorescent, their reactions to long waves are generally stronger than their reactions to short waves. Colorless to nearly colorless natural diamonds can have a weak to strong blue, yellow or orange fluorescence. The fluorescence of colorless to nearly colorless natural diamonds is generally uniformly distributed while the fluorescence distribution in a synthetic diamond is generally uneven. It follows the internal growth sectors of synthetic gemstones. Some sectors emit fluorescence, while others do not, so that a square or octagonal shape plus a cross-shaped pattern appears (see photos). Fluorescent bands parallel to the graining sometimes appear in individual growth areas.
Synthetics grown by conventional high pressure growth methods always phosphorescent for 60 seconds or more. The intensity of the phosphorescence is moderate to strong under short UV, and its color is usually yellow or greenish yellow. Colorless to almost colorless natural diamonds are phosphorescent sometimes in yellow under longwave UV and shortwave UV. Phosphorescence is generally weak and lasts 30 seconds or less.

© GIA

© GIA

Gemesis Corporation is an American manufacturer of synthetic diamonds. The company began selling yellow and processed colors for jewelry in 2002. Like other manufacturers, Gemesis is working on techniques to produce colorless synthetic diamonds in commercial quantities. In 2003, Apollo Diamond Inc., also an American manufacturer, announced the successful growth of synthetic diamonds large enough for jewelry by a technique that does not require high pressure and uses relatively modest temperatures of (730°C to 1130°C). The method (called chemical vapor deposition or CVD) deposits synthetic diamond from a carbon-rich gas onto a silicon or diamond surface. The synthetic diamond then grows to the surface in the form of a thin film. The final thickness of synthetic diamond depends on the time allowed for growth. Until recently, the synthetic diamond produced by CVD was not thick enough to produce fine jewelry stones. The maker has since exhibited 0.14 ct synthetic gems to 1.11 ct. Synthetic CVD diamonds range from pale brown to dark brown to gray and almost colorless. They may contain brown spots and grains as well as small, irregularly shaped black inclusions that may look like graphite. The stones are devoid of the molten metal inclusions common in high pressure synthetic diamonds. High pressure, high temperature (HPHT) annealing of brown CVD synthetics can make them light gray or colorless. Some CVD synthetic diamonds have a very weak orange fluorescence to yellow orange under longwave UV and weak to moderate orange to yellow-orange under shortwave UV. Others are inert. Unlike high pressure synthetic diamonds, CVD synthetics do not phosphorescent. Advanced testing is needed to separate them from other synthetic diamonds and, more importantly, natural diamonds...
Diamond treatments
Laser drilling : is an important treatment aimed at improving the clarity of the diamond (photo on the right). This results in straight laser drill holes that are easy to identify under magnification. Drill holes typically have a round cross section, while natural channels, which also extend inward from the surface of the diamond, are square, triangular, or hexagonal. Some treaters use a different laser technique that opens or widens a cleavage and helps to reach and whiten dark inclusions near the surface. The resulting feather (called ILD) looks more natural than a traditional laser drillhole. To identify this treatment, a microscope and various lighting techniques are used.
Fracture filling : is another treatment that improves clarity, and the flash effect is one way to detect it. To look for the flash effect, you need to use the magnification and reflected light to find where the fracture hits the surface. We then switch to lighting on a black background and visualize the presumed fracture filled in parallel to the fracture plane. We rock the stone repeatedly so that the background changes from light to dark. If the fracture is filled, two colors are visible and "blink". The effect occurs because the refractive index of the filler glass does not exactly match the refractive index of diamond for all wavelengths of light. Besides the flash effect, we can also see a cracked texture on the surface of the fill glass and gas bubbles trapped inside.
HPHT treatment : Treaters can remove the brown color from some diamonds through a treatment that combines high pressure and high temperature (HPHT). One of the effects of this treatment is the formation of graphite around the mineral inclusions. This is called graphitization. The cleavages of some HPHT treated diamonds appear glassy on the inside but grainy near the surface. Outward radiating stress cracks sometimes surround solid inclusions. HPHT treatment is most often used to make diamonds colorless. Most HPHT treated diamonds show no visible signs of treatment. The detection of this treatment often requires advanced tests in a gemological laboratory recognized as the GIA.
Irradiation : This is a brief exposure to electrons, neutrons, gamma rays, or a combination of both in a nuclear reactor. This treatment turns natural diamonds green and synthetic ones turn light yellow. Subsequent controlled heat treatment (annealing) changes the green to brownish orange. Sometimes this results in blue, orange and very rarely pink, purple or red colors. Unfortunately, it is difficult to predict which color will be processed each time. All green diamonds get their color from radiation exposure, but radiation can be natural or created in the laboratory. In many cases, even with sophisticated laboratory equipment and techniques, it is not possible to naturally separate natural green diamonds from processed green diamonds. An extremely dark green diamond color can be created by long exposure to radiation in a nuclear reactor. If you come across a diamond that is so dark green that it looks black, you can be pretty sure that it has been irradiated. Almost all naturally black diamonds are in fact extremely dark gray.
Since diamonds are generally more valuable than other stones, it is very important that any treatment, including irradiation, is detected and disclosed. This makes the testing process much more complex and time consuming. Most original diamond color testing should be done by a gemological lab. Spectrum testing is a way for a laboratory to detect radiation treatment. When cooled to extremely low temperatures, irradiated and annealed diamond can exhibit a spectrum with a thin line around 594 nm. Achieving sufficiently low temperatures requires the use of liquid nitrogen, which is why this test is usually performed in the laboratory. Before refining irradiation techniques, diamonds were bombarded with subatomic particles in a cyclotron. On these stones, magnification revealed low penetration and intense color zoning around the culet. Irradiation of a round gloss produces an umbrella-shaped pattern. Irradiation from the crown side causes areas of color to duplicate the faceted pattern that appears slightly below the surface. Today's diamond irradiation methods generally leave little or no color zoning. If color zoning is present, it is subtle and difficult to detect. A gemological microscope, diffuse transmitted light, and methylene iodide immersion can help find color zoning associated with irradiation. Natural and synthetic boron colored blue diamonds conduct electricity, while irradiated blue diamonds do not. Tests to separate the two are usually done in a gemological lab. Radioactivity is only detectable in certain irradiated diamonds. The improved irradiation techniques used today leave no measurable radioactivity behind.
Natural simulants
Synthetic simulants
Synthetic rutile : Also has a high refractive index, but it is also birefringent and shows extreme double refraction. Synthetic rutile has a subadamantine to submetallic luster and extreme fires. Its spectrum, which shows a cutoff around 430 nm, distinguishes it from diamond and other simulants. A thermal tester reports that synthetic rutile is not diamond.
CZ diamond (cubic zirconia) : as diamond has a high refractive index and is isotropic because it is cubic, it cannot therefore be identified by its optical properties alone. But CZ has a subadamantine luster, a higher density of 5.80, and a higher dispersion which gives it bigger fires than diamond. CZ is usually without inclusion, but magnification sometimes reveals gas bubbles and unmelted zirconium oxide. If we look at the pavilion of a round-shiny CZ on a black background and tilt it repeatedly, most of the facets show orange flashes. CZ fluoresces greenish yellow or yellowish orange under long UV and yellow under short UV. A thermal tester indicates that CZ is not a diamond.
Synthetic sapphire : has a refractive index of 1.76 to 1.77, birefringence of 0.008 and uniaxial optical character easily separates it from diamond and other colorless materials. Synthetic sapphire has a polished glassy to subadamantine luster and low fires. It is inert or emits a weak bluish-white fluorescence under short UV. Magnification and fluorescence can separate colorless synthetic sapphire from its natural counterpart.
Synthetic spinel : is like unirefringent diamond, but its refractive index of 1.73 sets it apart. In addition, the synthetic spinel has a polished glassy to subadamantine luster and weak fires. The polariscope reveals ADR and hatching. Synthetic spinel fluoresces a moderate to strong chalky blue or strong greenish blue under short UV and sometimes weak green under long UV. Natural colorless spinel is very rare.



© GI

Less common synthetic simulants
Less common diamond simulants are strontium titanate, gadolinium gallium garnet (GGG), yttrium aluminum garnet (YAG), synthetic quartz, and artificial glass.
Strontium titanate : Has a high refractive index and is unirefringent like diamond, but its polished glassy to subadamantine luster, extreme fires, and low thermal conductivity set it apart.
GGGs and YAGs : These synthetic garnets have high refractive indexes and isotropic, their luster is glassy to subadamantine, and both register as non-diamond with a thermal tester, but some differences help you separate them from one another. The fires of the GGG are moderate, while those of the YAGs is weak. GGGs fluorescence is moderate to strong pink orange under shortwave UV, while YAGs are inert to moderate orange under longwave UV and inert to weak orange under shortwave UV. The density of YAGs is 4.50 to 4.60, while that of GGGs is 7.05. YAG's pavilion flash is blue and purple on most facets, while GGG's is orange and blue, but this test only works with well-proportioned round brilliants.
Synthetic quartz : is typically cultivated for use in electronic devices, but some have marketed it as a diamond simulant. Quartz's low refractive index (1.54-1.55) and birefringence make it easy to distinguish from diamond and other colorless materials, but not from its natural counterpart.
Artificial glasses : are unirefringent, and of a lower refractive index than that of diamond (1.47 to 1.70), the vitreous luster and the weak to nonexistent fires prove that it is not a diamond. Magnification can reveal gas bubbles, surface cavities and flow lines (features completely absent from diamond). High lead glass, used in fashion jewelry, has a higher refractive index and more fires than lead free glasses.
The assembled simulants
Assembled stones that mimic diamond include strontium titanate doublets coupled with colorless synthetic sapphire or synthetic spinel. If a separation plane at or below the belt is present, this is proof that it is assembled. If further tests are inconclusive, it is possible to prove the assembly by finding the differences in refractive index between the crown and the bell.
Some doublets use the diamond as the crown and a diamond simulant as the pavilion. If you only touch the crown with a thermal tester, it will accurately indicate "diamond". To avoid making this mistake, one should use the thermal tester on the horn, then look for a separation plane under magnification or check with a UV lamp to see if the crown and horn fluoresce differently.
Hardness : 10
Density : 3.50 to 3.53
Fracture : Irregular
Streak : White
TP : Transparent to opaque
RI : 2.435
Birefringence : 0
Optical character : None
Pleochroism : None
Fluorescence : Blue, green, orange
Solubility : Insoluble
Magnetism : Diamagnetic
Radioactivity : None