How to identify a mineral ? - Part 1
Introduction :
Many will say that there are simple tests to identify minerals, which is unfortunately totally wrong... The most powerful asset in sample recognition is above all the experience and strong geological knowledge of process of crystal formations and their contexts. But while important this asset is not always enough to break the mystery of a blank on a label...
When a collector finds, buys or trades a specimen, his first concern is not to damage it and keep it as best as possible. The tests "basic" indicated in the books and relayed widely on the forums will almost all alter the specimen, being also very relative and subjective... We will resume all these tests.
Hardness :
Mineralogical species can be classified on a scale of hardness called the Mohs scale. It represents the resistance to scratching. It is a relative scale from 1 to 10 and is almost linear from 1 to 9, because the diamond, much harder (10 hardness) is actually 122 times harder than the corundum that is 9.
These tests of "who scratches who ?" and "who is scratched by whom ?" are very random, they also damage your sample. They depend on the pressure exerted during the streaking and the orientation of your crystal. The hardness of minerals is not the same in the 3 directions of space, this problem is well known lapidaries who carve black diamonds for example... You can also deal with a rock made up of several minerals, a piece of lapis lazuli for example contains both lazurite (hardness 5), calcite (3), sodalite (6) and pyrite (6.5).
Density :
Requires the mass of the sample and its volume or the mass of the sample in the air and then in the water. In other words, the measurement of density passes through a series of simple weighings and calculations.
Important inaccuracies will be grafted on the result, especially if the sample is small. In addition, the larger the sample, the less homogeneous it is (inclusions, presence of other minerals, etc...). Finally, the density of a mineralogical species can vary considerably depending on its chemical composition.
On the other hand, immersing a specimen that is not known in the water can damage it. Some mineralogical species are soluble in water (halite, hanksite, etc...), while others are corroded by the chemical elements contained in the water (eg. the pyromorphites are dissolved by the chlorine contained in the water). tap water, carbonates are attacked by H+ ions...).

Streak :
It is the color of the mineral reduced to powder (or the color that will leave the mineral if it is rubbed upside down of a porcelain plate for the minerals of low hardness). The color of the streak is not necessarily the same as that of the mineral.
It is a destructive test or at least will spoil the sample. On the contrary, it is easy on specimens intended to be cut or processed, because sawing or grinding reduces parts of the sample in powder.
Color :
Color is an important parameter in the identification of a mineral and easy to appreciate, however it is also a source of great mistakes... The best example is probably the ruby of the English crown which is in fact... a spinel... Spinel and ruby are two red minerals that we meet associated in the same deposit...
The minerals are classified in 3 main categories :
- Achromatic : these are colorless minerals (hyaline quartz).
- Idiochromatic : these are the minerals whose color is typical of the atoms they contain.
- Allochromatic : these are the minerals whose color is linked to the trace elements (impurities) contained in the crystal lattice of the mineral.
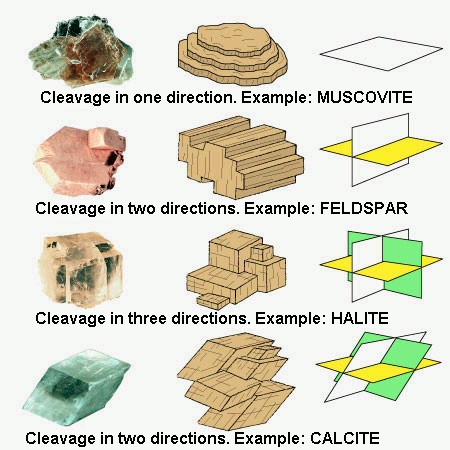
Cleavage :
Or preferential fracture plane. Its orientation is directed by the crystalline structure. There may be up to 4 cleavage planes in a crystal (eg. fluorite). We classify the minerals according to the quality of the cleavage (from excellent to bad), which is very subjective when we do not use this parameter very regularly. On the other hand, the number and orientation of the cleavage (s) constitutes interesting information. They make it possible to distinguish between an amphibole and a pyroxene (two black prismatic minerals that both have 2 cleavage planes) : the pyroxene cleavage planes intersect at 90 ° while those of an amphibole overlap with 120 °...

The fracture :
According to different works, up to 6 different types of fracture can be distinguished. The only breakout really used is easy to spot is the conchoidal (or shell-shaped) break, it is the typical break of glass, but also quartz, flint, obsidian.
Luster :
This is the reflectivity of a material (ratio of the amount of reflected light to the amount of light received). The visual perception of how a mineral reflects light is called the luster. Minerals are classified into two main types : metallic and non-metallic. These 2 categories include subcategories whose determination for an uninitiated eye is difficult.
In the photo, cubes of metallic pyrite associated with non-metallic quartz.
Morphology :
It's the shape of the crystal. A mineral can take many forms, its crystals can be eroded, dissolved, or replaced by another species (pseudomorph). Morphology is very important for a determination, the crystalline faces are unfortunately not always well expressed, the morphology does not always allow a determination.

Fluorescence :
It is the property of a mineral to emit visible light when subjected to a UV light source. The color of fluorescence can bring information on a species, but especially on its deposit. The same mineral may indeed have different fluorescence color depending on its locality. There are 2 main types of UV waves to observe fluorescence hue, so-called short waves, and long waves. The fluorescent tints vary depending on the UV source used.
It should be noted that some minerals are also fluorescent when heated, this phenomenon of "thermoluminescence" is difficult to observe without damaging the sample.
Radioactivity :

It is a natural physical phenomenon in which unstable atomic nuclei spontaneously transform ("disintegrate") by releasing energy in the form of various radiations, to transform themselves into more stable atomic nuclei by losing some of their their mass. It is measured with a Geiger counter.
Too few common mineralogical species are radioactive for the Geiger counter to be an attractive investment. Except of course if you base a collection on this theme.

Magnetism :
It is the ability of a mineral to be attracted to a magnet. It is easy to test without damaging the specimen, however, few specimens are magnetic...
The smell and taste :
Some minerals have a characteristic odor, including sulphur or some sulfides. In the same way it may be interesting to spend very slightly the language on a specimen to appreciate (or not) the taste (halite, hanksite, coquimbite, etc...). It will always spit and rinse the mouth after tasting a specimen, some species are toxic.
Acid test :
It consists of spilling a few drops of hydrochloric acid (or white vinegar) on the sample. When effervescence occurs this test is used to identify carbonates.
This test is very likely to damage your specimen, even if no effervescence occurs...
Refractive index :
The refractive index is a dimensionless value characteristic of material, describing the behavior of light in this material.
It is measured in transparent to opaque materials with a refractometer. It is a characteristic mineral value that allows identification. It is widely used in gemology to identify gemstones. It is unfortunately a fairly expensive device... Count around 600€ for a good quality refractometer.

Pleochroism :

Very easy to highlight for transparent to translucent minerals with a dichroscope or a polarizing filter. Cubic or amorphous minerals are not pleochroic. If you find the dichroscope expensive, you can also easily see if your crystal changes color depending on its orientation using a polarizing filter available at photo dealers. If the English had sought to highlight the pleochroism of the "ruby" of the English crown, they would have seen it was a spinel...
This test is very often forgotten and yet easy to set up and non-destructive !
Conclusion :
Although interesting, these tests do not always make it possible (and without any experience) to identify a mineralogical specimen. One can wonder then how do collectors of micro-minerals to identify their finds. This will be treated in a second part : laboratory identification !