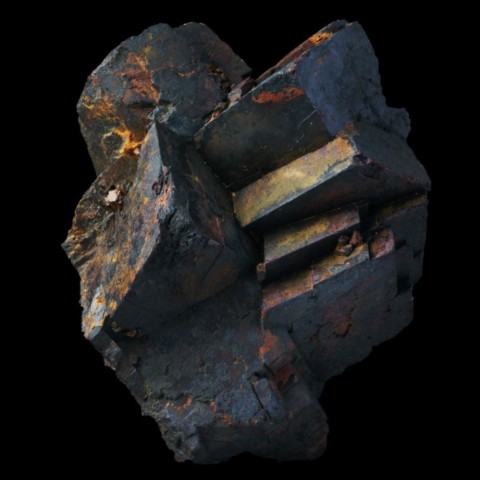LIMONITE
Class : Oxides and hydroxides
Subclass : Hydroxides
Crystal system : -
Chemistry : Fe2O3 3H2O
Rarity : Ubiquitous
The name limonite originally designated an iron hydroxide with the supposed formula Fe2O3 H2O. In fact no mineral meeting this formula could be synthesized and scientific research revealed that the majority of "limonite" samples were actually cryptocrystalline goethite with capillary or absorbed water. Currently, the term limonite nevertheless continues to be a convenient global designation to designate poorly defined products predominantly iron hydrates. From a mineralogical point of view, limonite usually designates a mixture in variable proportions of different minerals having crystallized from hydrated ferric oxide gels (essentially goethite, pure or dominant, commonly lepidocrocite or hematite), associated to variable quantities of clays, carbonates, colloidal silica, etc... Crystallinity extends from colloidal terms to well-crystallized products. For all these reasons, its physical and chemical properties fluctuate enormously. It very frequently forms on the surface by oxidation of iron-containing minerals (pyrite, siderite, chalcopyrite, but also biotite, pyroxene or amphibole) to which it gives a characteristic rusty color. It is particularly abundant in the oxidized part of the iron sulphide deposits, constituting the "gossans" sometimes more than 100 meters thick. Its name derives from the Greek leimon (meadow) or the Latin limus (mud, vase) in allusion to the frequent formation of limonite in marshy areas, we find this origin in the old French name "marsh iron" which designated it. Limonite constitutes compact, fibrous, hilly or concretionary masses (oolites and stalactites), often earthy or powdery, with a density varying from 2.7 to 4.3 and a hardness from 4 to 5.5, with a variable water content but often high. It is an important iron ore.
Main photo : Limonite from Montroc, Tarn, France
Limonite in the World
Twinning
No twins known for this mineral species, but limonite can easily replace pyrite or siderite (pseudomorph); we also speak of "limonitized" pyrites.
Fakes and treatments
No fakes recorded for this mineral species.
Hardness : 4 to 5.5
Density : 2.7 to 4.3
Fracture : Irregular, fibrous, conchoidal
Streak : Variable
TP : Opaque
RI : -
Birefringence : -
Optical character : -
Pleochroism : None
Fluorescence : None
Solubility : Insoluble
Magnetism : NoneRadioactivity : None